Cell & Gene Therapy
Cell & Gene Therapy
Chimeric Antigen Receptor (CAR) cell therapies have emerged as an effective method in immuno-oncology research for successfully treating some forms of leukemia and lymphoma. However, performing accurate cell analysis, including precise size determination and viability, throughout the process remains inefficient and cumbersome, currently requiring the use of multiple instruments.
Moxi GO II covers the full CAR-T research workflow.
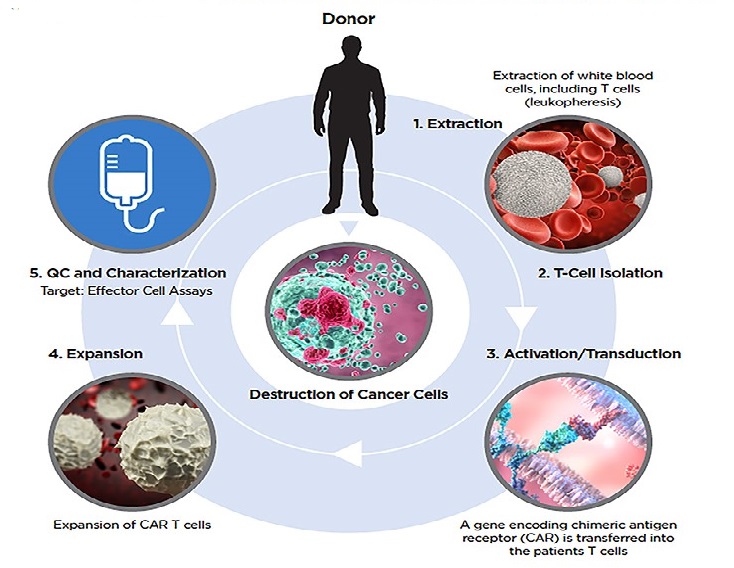
The CAR-T workflow involves Extraction of the patients T cells Isolation, Activation/Transduction, Expansion, and QC and Characterization. The Moxi GO II is the ideal platform for each step in the CAR-T research workflow.
1.Extraction
- Isolated PBMC viability check
2.T-Cell Isolation
- Purity Check
3.Activation/Transduction
- QC of Count
- Dynabead count, pre- and post-activation
- Activation monitoring
- Transduction efficiency monitoring and optimization
4.Expansion
- Total count, viability, MCV, Phenotypes, T:E Ratios
5. QC and Characterization
- Effector Cell Count, MCV and viability
- Target: Effector Cell Assays
- Cell Health
The Moxi GO II Cell Analyzer perfectly addresses all of the cell analysis needs required in CAR research, whether you’re performing basic research or in full production. The Moxi GO II is the only instrument in the world that combines Coulter Principle (electronic) cell detection with simultaneous two-color fluorescence detection. This unique combination allows for exact volumetric cell sizing (<3% CV), which is required to determine the end of the Expansion phase, while also allowing for the analysis of fluorescence-based viability, GFP expression, immunophenotyping , etc.
